CBD product receives positive safety assessment from FSA
In Business news
Follow this topic
Record learning outcomes
A cannabidiol (CBD) product has, for the first time, received a positive safety assessment from the Food Standards Agency.
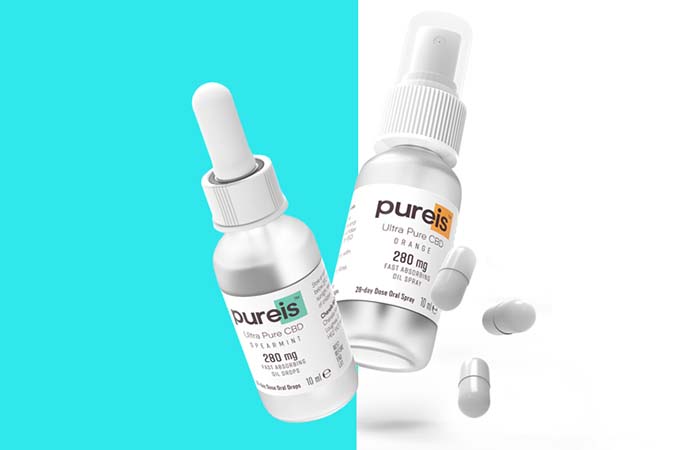
Until now no CBD food supplement in the UK has received this endorsement. However, the FSA has concluded that supplier Chanelle McCoy Health has provided sufficient information to assure its Pureis Ultra Pure CBD is safe as a food supplement.
This endorsement from the FSA Scientific Committee indicates that the brand has successfully fulfilled the criteria necessary to progress to the final stage of the Novel Food Licensing process.
CBD is classified as a Novel Food, which refers to food products that are either entirely new or produced using methods not previously used for human consumption. CBD products fall into this category as they are a relatively recent addition to the mainstream food market.
The UK’s FSA is the first authority globally to conclude and publish safety assessments for CBD food supplement products.
Pureis Ultra Pure CBD claims to be the world’s leading brand of lab-made CBD food supplements which contain no THC, the principal psychoactive constituent of plant-derived cannabis.
“This is a monumental achievement and a complete game-changer for the CBD industry,” according to Chanelle McCoy, chief executive of Chanelle McCoy Health.
Since setting up the company four years ago, the founders have developed a wide portfolio of lab-made CBD in oral food supplements and topical treatments, which are currently being sold through pharmacies and other UK outlets.